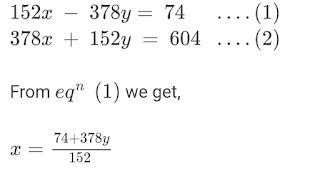Ques 1: If a trait A exists in 10% of a population of an asexually reproducing species and a trait B exists in 60% of the same population, which trait is likely to have arisen earlier? Ans 1: In asexual reproduction, the reproducing cells produces a copy of their DNA through some special chemical reactions. However, this copying of DNA is not completely accurate and therefore, the newly formed DNA has some variations. It can be easily seen in the above figure that in asexual reproduction, very few variations are allowed. Therefore, if a trait is present in only 10% of the total population, it is more likely that the trait has arisen recently. Hence, it can be concluded that trait B that exists in 60% of the same population has arisen earlier than trait A. Ques 2: How does the creation of variations in a species promote survival? Ans 2: Sometimes for a species, the environmental conditions change so drastically(badly) ...