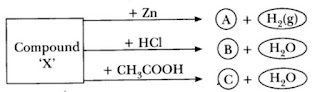
The above reaction is an example of a: (a) combination reaction. (b) double displacement reaction. (c) decomposition reaction. (d) displacement reaction.

Ques 2: The above reaction is an example of a: Fe 2 O 3 + 2 Al → Al 2 O 3 + 2 Fe (a) combination reaction. (b) double displacement reaction. (c) decomposition reaction. (d) displacement reaction. Ans 2: (d) The given reaction is an example of a displacement reaction.