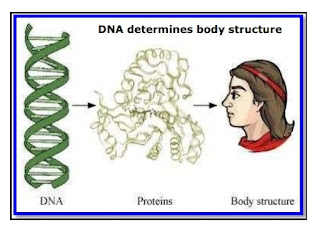
Ques 1: What is the importance of DNA copying in reproduction? Ans 1: DNA (Deoxyribonucleic acid) is the genetic material found in the chromosomes, present in the nucleus of the cell. The DNA (Deoxyribonucleic acid) is the information site for making proteins and each specific type of protein leads to the specific type of body design. Thus, it is the DNA molecule that specifies the body design of an individual. Therefore, it can be concluded that it is the DNA that gets transferred from parents to their offsprings and makes them look similar. Ques 2: Why is variation beneficial to the species but not necessarily for the individual? Ans 2: Variations are beneficial to the species than individual because s sometimes for a species, the environmental conditions change so drastically that their survival becomes difficult. For example, if the temperature of water increases suddenly, then most of the bacteria living in that wate...


Comments
Post a Comment