S-Block Elements || S-block element class 11 chapter 8 || Group 1A, 2A elements || S block elements chapter 8 notes class 11 || Chemistry ||
INTRODUCTION :
The s- block elements of the periodic table are those in which the last electron enters into the outermost s-orbital.
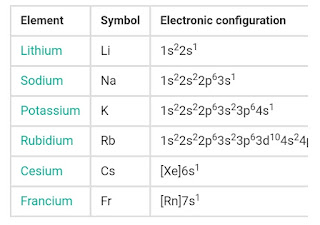
As the s-orbital can accommodate only two electrons, in two group (1 & 2) belong to the s-block of the periodic table. Group 1 of the periodic table consists of the elements : lithium (L i), sodium (Na), potassium (K), rubidium (R b), caesium (C s) and francium (F r). They are collectively known as alkali metals. These are called because they forms hydroxides on reaction with water which are strongly alkaline in nature.
The elements of Group 2nd include beryllium (Be), magnesium (Mg), calcium (C a), strontium (S r), barium (B a) and radium (Ra). These elements with the exception of Beryllium are commonly known as the alkaline earth metals. These are so called because their oxide and hydroxide are alkaline in nature and these metal oxides are found in the earth’s crust .
The general electronic configuration of s- block element is [ noble gas] n s¹ for alkali metal and [noble gas]n s² for alkaline earth metals. Lithium and beryllium, which are the first elements of Group 1 and Group 2 respectively exhibit some properties which are different from those of the other members of the respective group. In these anomalous properties they resembles the second element of the next group. Thus, lithium show similarities with magnesium and beryllium to aluminium in many of their properties. This type of diagonal similarity is commonly referred to as the diagonal relationship in the periodic table. The diagonal relationship is due to the similarities in ionic size or (charged /radius) ratio of the elements. The general electronic configuration of s- block element is [ noble gas] n s¹ for alkali metal are:
The general electronic configuration of s- block element is [ noble gas] n s² for alkaline earth metals are:Electronic Configuration of s block element:
All the alkali metal have only one valence electron, n s¹ outside the noble gas core. The single valence electron is at a long distance from the nucleus and is only weakly held. Hence, the loosely held s-electrons in the outermost valence shell of these elements make them the most electropositive metals. They readily loses electron to form monovalent M+ ions. Hence they are never found in free state in nature. Alkaline earth metals have two electrons in the s-orbital of their valence shell. The compounds of these elements are also pre dominantly ionic. The genera
Atomic and Ionic Radii :
The alkali metal atoms have the largest size in a particular period of the periodic table. The atomic and ionic radii of alkali metal increase with moving down the group i.e., there is increase in the size while going from Lithium(L i) to Caesium(C s).
The atomic and ionic radii of the alkaline earth metals are smaller than those of the corresponding alkali metals in the same period. This is due to the increased nuclear charge in these elements. Within the group, the atomic and ionic radii increase with increase in atomic number due to the increase in number of atomic shells.
Ionisation Enthalpy :
The ionisation enthalpy of the alkali metals are considerably low and decrease down the group from L i to C s. This is because the effect of increasing size outweighs the increasing nuclear charge and the outermost electron is very well screened from the nuclear charge. The second ionisation energy is extremely high because of the removal of 2nd electron from a stable noble electron configuration of monovalent metal cation. The alkaline earth metals have low ionisation enthalpy due to fairly large size of the atoms. Since the atomic size increases down the group, their ionisation enthalpy decreases. The first ionisation enthalpy of the alkaline earth metals is higher than those of the corresponding alkali metals for the same period. This is due to their smaller size as compared to the corresponding alkali metals. The second ionisation enthalpy of the alkaline earth metals are smaller than those of the corresponding alkali metals as in the alkali metals the second electron is to be removed from an inert gas electronic configuration.
Hydration Enthalpy:
The hydration enthalpy of alkali metals ions decrease with increase in ionic sizes. L i+ has maximum degree of hydration and for this reasons lithium salts are mostly hydrated e.g., L i Cl . 2H2O. Like alkali metal ions hydration enthalpy of alkaline earth metal ions decrease with increase in ionic size down the group.
Be²+ > Mg²+ > C a²+ > S r²+ > B a²+
The hydration enthalpy of alkaline earth metal ions are larger than those of alkali metal ions. Thus, compounds of alkaline earth metals are more extensively hydrated than those of the alkali metals, e.g., Mg Cl 2 and C a Cl 2 exist as Mg Cl 2 .6H2O and C a Cl 2 . 6H2O while Na Cl and K Cl do not form such hydrates.
Physical properties:
All the alkali metal are silvery white, soft (because of having only one valency electron which participate in the bonding) and light metals. Because of their larger size, these element have low density which increases down the group from L i to C s. However, potassium is lighter than sodium because of the larger atomic volume. The melting and boiling point of the alkali metals are low which indicate weak metallic bonding due to the presence of only a single valence electron in them. The strength of the metallic bond decreases down the group, and the melting point decreases accordingly. The melting point of lithium is nearly twice as high as that for sodium because of the strong metallic bonding on account of its small size. However, the melting points of the other all close together. The alkali metals and their salts impart characteristic colour to an oxidizing flame. This is because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electrons come back to the ground state, there is an emission of radiation in visible region.
These elements when irradiated with light, the light energy absorbed may be sufficient to make an atom lose electron. This property make caesium and potassium useful as electrodes in photoelectric cell. The alkaline earth metals, in general, are silvery white, lustrous and relatively soft but harder than the alkali metals. Beryllium and magnesium appear to be somewhat greyish. The melting and boiling point of these metals are higher than alkali metals due to smaller size and two valence electrons. Because of the low ionisation enthalpy they are strongly electropositive in nature. The electropositive character increases down the group from Be to B a. Calcium, strontium and barium impart characteristic colour to the flame.
The electrons in beryllium and magnesium are strongly bounded to get excited by flame. Hence these elements do not impart any colour to the flame. Like alkali metal, electrical and thermal conductivity of the alkaline earth metal are high.
Chemical Properties:
The alkali metal are highly reactive due to their larger size and low ionisation enthalpy. The reactivity of these metal increases down the group. The alkaline earth metals are less reactive than the alkali metals. The reactivity of these elements increases on going down the group.
» Reactivity towards air and water : The alkali metals tarnish in dry air due to the formation of their oxides which in turn react with moisture to form hydroxides. They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxide. The superoxide O2 – ion is stable only in the presence of larger cation such as K, R b,C s.
All five metals can be induced to form the normal oxide, peroxide or superoxide by dissolving the metal in liquid ammonia and bubbling in the appropriate amount of oxygen. Lithium show exceptional behaviour in reaction directly with nitrogen of air to form the nitride, L i 3 N. L i 3 N is ionic (3Li+ and N3–), and is ruby red.
The alkali metals react with water to form hydroxide and dihydrogen. The reaction becomes increasingly violent on descending the group.Although lithium has most negative electrode potential (E) value, its reaction with water is less vigorous than that of sodium which has the least negative electrode potential (E) value among the alkali metals. This behaviour of lithium is attributed to its small size and very high hydration energy. Other metals of the group react explosively with water.
2M + 2H2O → 2MOH + H2
(M = alkali metal cation).
They also react with proton donors such as alcohol, gaseous ammonia and alkynes. Because of their high reactivity towards air and water, the alkali metals are normally kept in kerosene oil. Beryllium and magnesium are inert to oxygen and water because of the formation of an oxide film on their surface. However, powdered beryllium burns brilliantly on ignition in air to give Be O and Be 3 N2. Magnesium is more electropositive and burns with dazzling brilliance in air to give Mg O and Mg 3 N2. Calcium, strontium and barium are readily attacked by air to form the oxide and nitride. They also react with water with increasing vigour even in cold to form hydroxides.
» Reactivity towards dihydrogen : The alkali metals react with dihydrogen at about 673 K (lithium at 1073 K) to form hydrides. All the metal hydrides are ionic solid with high melting points . All alkaline earth metals except beryllium combine with hydrogen upon heating to form their hydrides, M H2, which are also high melting solids. Be H2, however, can be prepared by the reaction of Be Cl 2 with
L i Al H 4 . 2BeCl2 + L i Al H 4 → 2 Be H2 + Li Cl + Al Cl 3
» Reactivity towards halogens : The alkali metals readily react vigorously with halogens to form ionic halide , MX. However lithium halides are some what covalent. It is because of the high polarisation capability of lithium ion. The L i+ ion is very small in size and has high tendency to distort electron cloud around the negative halide ion. Since anion with larger size can be easily distorted , among halides lithium iodide is the most covalent in nature.
All the alkaline earth metals combine with halogens at elevated temperature forming their halides.
M + X2 → M X2
(X = F, Cl, B r, l)
» Reducing nature: The alkali metals, are the strong reducing agents, lithium being the most and sodium being the least powerful with the small size of its ion, lithium has the highest hydration enthalpy which account for its high negative E value and its high reducing power. Like alkali metals, the alkaline earth metals are strong reducing agents. This is indicated by larger negative value of their reduction potentials. Beryllium has less negative value compared to other alkaline earth metals due to relatively large value of the atomisation enthalpy of the metal. However, its reducing nature is due to large hydration energy associated with the small size of Be 2+ ion.
» Solution in liquid ammonia : The alkali metals dissolve in liquid ammonia giving deep blue solutions.
The blue colour, corresponding to a broad absorption band near 1500 nm that falls into the visible range, is attributed to the solvated electron. These dilute solutions conduct electricity better than any salt in any liquid and the conductivity is similar to that of the pure metals. Conduction is due mainly to the presence of solvated electrons. While the life time of the solvated electron in water is very short, in very pure liquid ammonia it may be quite long ( < 1% decomposition per day).As the solutions are made more concentrated, the molar conductivity at first decreases, reaching a minimum at about 0.05 molar ; thereafter, it increases again until in saturated solutions it is comparable to that of the metal. According to more satisfactory model of the solvated electron, the electron is not localised but is “smeared out” over a large volume so that the surrounding molecules experience electronic and orientational polarisation. The electron is trapped in the resultant polarisation field, and repulsion between the electron and the electrons of solvent molecules leads to the formation of a cavity (of diameter approximately 3.0–3.4 Å) within which the electron has the highest probability of being found. This cavity concept is based on the fact that these dilute solutions are of much lower density than pure solvent, that is, they occupy far greater volume than that expected from the sum of the volumes of metal and solvent.
Very dilute solutions of the metals are paramagnetic, with approximately one unpaired electron per metal atom (corresponding to one solvated electron per metal atom) ; this paramagnetism decreases at higher concentration. As the concentration of metal increases, metal ion clusters are formed.Above 3M concentration the solutions are diamagnetic and, no longer blue but are bronze/copper-bronze coloured with a metallic luster.
The blue solutions of alkali metals in liquid ammonia, decompose very slowly with liberation of hydrogen (i.e. reduction of the solvent).
GENERAL CHARACTERISTICS OF THE COMPOUNDS OF THE ALKALI METALS AND ALKALINE EARTH
» METALS :
The oxides and the peroxides are colourless when pure, but the super oxides are yellow or orange in colour. Li O2 and Na O2 are yellow, K O2 and C s O2 are orange whereas R b O2 bromide is brown in colour. The super oxides are also paramagnetic. The hydroxide are all white crystalline solids. The alkali metal hydroxides are the strongest of all bases and dissolve freely in water with evolution of much heat on account of intense hydration.
The melting and boiling points of alkali metal halides always follow the trend : fluoride > chloride > bromide> iodide. All alkali metal halides are generally soluble in water. The low solubility of Li F in water is due to its high lattice enthalpy whereas the low solubility of Cs I is due to smaller hydration enthalpy of its two ions. Other halides of lithium are soluble in ethanol, acetone and ethyl acetate; L i Cl is soluble in pyridine also.The alkali metals form salts with all the oxo-acids. The thermal stability of oxy-acid salts generally increases down the group with increasing metallic character, i.e. electropositive character. They are generally soluble in water and thermally stable. Their carbonates (M2 CO 3 ) and in most cases the hydrogen carbonates (MH CO 3) also are highly stable to heat.As the electropositive character increases down the group, the stability of the carbonate and hydrogen carbonates increases. Because group 1 metals are so strongly basic, they (except lithium) also form solid bicarbonates. No other metals form solid bicarbonates. Lithium carbonate is not so stable to heat. Its hydrogen carbonate does not exist as a solid. Although NH 4 H CO 3 also exists as a solid. The crystal structures of Na H CO 3 and K H CO 3 both show hydrogen bonding, but are different.
(a) In Na H CO 3, the H CO 3–ions are linked into an infinite chain and (b) in K H CO 3, H CO 3– forms a dimeric anion.
The solubility of the alkali metal salts except fluorides, carbonates and hydroxides decreases down the group from L i to C s. This is because of the fact that down the group with increasing size of cation the lattice energy as well as hydration energy also decrease but the change in hydration energy is more as compare to that of lattice energy.
MO + H2O → M(OH)2
The solubility, thermal stability and the basic character of these hydroxides increase with increasing atomic number from Mg(OH)2 to B a (OH)2 . The alkaline earth metal hydroxides are however, less basic and less stable than alkali metal hydroxides. Beryllium hydroxide is amphoteric in nature as it reacts with acid and alkali both.
Be(OH)2 + 2OH → [Be(OH)4 ]
Be(OH)2 + 2HCl + 2H2O → [Be(H2O)4 ]Cl 2 .
The anhydrous halides of alkaline earth metals are polymeric .Except for beryllium halides, all other halides of alkaline earth metal are ionic in nature. Beryllium halides are essentially covalent and soluble in organic solvents. Beryllium chloride vapour contains Be Cl 2 and (Be Cl 2 ) 2 but the solid is polymerised.
In the vapour phase Be Cl 2 tends to form a chloro-bridged dimer which dissociates into the linear monomer at high temperature of the order of 1200 K. The tendency to form halide hydrates gradually decreases (for example, Mg Cl 2 .6H2O, Ca Cl 2 .6H2O, S r Cl 2 .6H2O and Ba Cl 2




Comments
Post a Comment