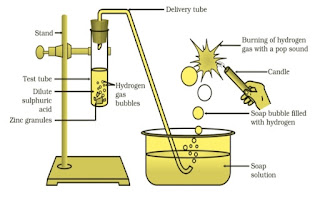
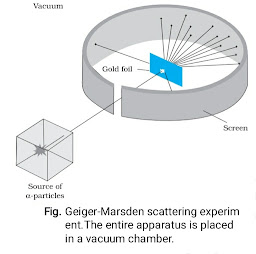
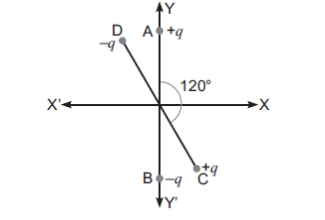
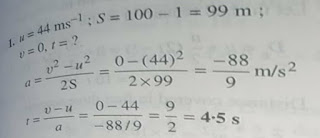Dimensional formula checking for v²= 2 g h
» Checking dimensional correctness for v²= 2 g h LHS = v² = [L T ^-1]² = [L² T ^-2] RHS = 2 g h = 2 [L T ^-1][L] = [L² T^-2] »LHS = RHS So, the LHS= RHS. HENCE, the given formula is dimensionally correct. » Checking dimensional correctness for v = u+ at LHS = v = [L T ^-1] RHS = u + at= [L T ^-1] + [L T^-2][T] = [L T^-1] »LHS = RHS So, the LHS= RHS. HENCE, the given formula is dimensionally correct.