ATOMS || THE BASIC COMPONENT OF MATTER || Notes ||
1. Introduction
Atoms are the basic component of matter. It consists of many sub atomic particles. In 1897, the experiments on electric discharge through gases carried out by the English physicist J. J. Thomson (1856 – 1940) revealed that atoms of different elements contain negatively charged
constituents (electrons) that are identical for all atoms. However, atoms on a
whole are electrically neutral. Therefore, an atom must also contain some positive charge to neutralise the negative charge of the electrons.The first model of atom was proposed by J. J. Thomson in 1898.
According to this model, the positive charge of the atom is uniformly
distributed throughout the volume of the atom and the negatively charged
electrons are embedded in it like seeds in a watermelon. This model was
picturesquely called plum pudding model of the atoms. The distribution of the electrons and positive charges are very different from that proposed in this model. We know that condensed matter (solids and liquids) and dense gases at all temperatures emit electromagnetic radiation in which a continuous distribution of several wavelengths is present, though with different intensities. This radiation is considered to be due to oscillations of atoms and molecules, governed by the interaction of each atom or
molecule with its neighbours. In contrast, light emitted from
rarefied gases heated in a flame, or excited electrically in a
glow tube such as the familiar neon sign or mercury vapour light has only certain discrete wavelengths. The spectrum
appears as a series of bright lines. In such gases, the
average spacing between atoms is large. Hence, the radiation emitted can be considered due to individual atoms rather than because of interactions between atoms or molecules.
Ernst Rutherford (1871–1937), a former research
student of J. J. Thomson, was engaged in experiments on
α-particles emitted by some radioactive elements. In 1906, he proposed a classic experiment of scattering of these α-particles by atoms to investigate the atomic structure.
This experiment was later performed around 1911 by Hans Geiger (1882–1945) and Ernst Marsden (1889–1970, who was 20 year-old student and had not yet earned his bachelor’s degree). The explanation of the results led to the birth of Rutherford’s planetary model of atom (also called the nuclear model of the atom). According to this the entire positive charge and most of the mass of the atom is concentrated in a small volume called the nucleus with electrons revolving around the nucleus just as planets revolve around the sun. Rutherford’s nuclear model was a major step towards how we see
the atom today. However, it could not explain why atoms emit light of
only discrete wavelengths. How could an atom as simple as hydrogen, consisting of a single electron and a single proton, emit a complex spectrum of specific wavelengths? In the classical picture of an atom, the electron revolves round the nucleus much like the way a planet revolves round the sun. However, we shall see that there are some serious difficulties in accepting such a model.
2. ALPHA-PARTICLE SCATTERING AND
RUTHERFORD’S NUCLEAR MODEL OF ATOM
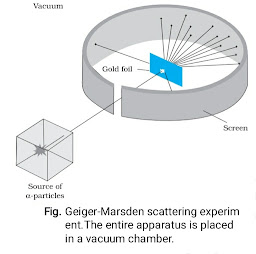
At the suggestion of Ernst Rutherford, in 1911, H. Geiger and E. Marsden
performed some experiments. In one of their experiments, as shown in the figure given below they directed a beam of
5.5 MeV α-particles emitted from a
214
83Bi radioactive source at a thin metal foil made of gold. A schematic diagram of this experiment. Alpha-particles emitted by a 214
83Bi
radioactive source were collimated into
a narrow beam by their passage
through lead bricks. The beam was allowed to fall on a thin foil of gold of thickness 2.1 × 10–7 m. The scattered alpha-particles were observed through
a rotatable detector consisting of zinc
sulphide screen and a microscope. The scattered alpha-particles on striking the screen produced brief light flashes or scintillations. These flashes may be viewed through a microscope and the distribution of the number of scattered
particles may be studied as a function of angle of scattering.
A typical graph of the total number of α-particles scattered at different
angles, in a given interval of time. The dots represent the data points and the solid curve is the theoretical prediction based on the assumption that the target atom has a small, dense, positively charged nucleus. Many of the α-particles pass through the foil. It means that they do not suffer any collisions. Only about 0.14% of the incident α-particles scatter by more than 1°; and about 1 in 8000 deflect by more than 90°. Rutherford argued that, to deflect the α-particle backwards, it must experience a large repulsive force. This force could be provided if the greater part of the
mass of the atom and its positive charge were concentrated tightly at its centre. Then the incoming α-particle could get
very close to the positive charge without
penetrating it, and such a close encounter would result in a large deflection. This agreement supported the hypothesis of the nuclear atom. This is why Rutherford is credited with the
discovery of the nucleus.
In Rutherford’s nuclear model of the atom, the entire positive charge and most of the mass of the atom are concentrated in the nucleus with the electrons some distance away. The electrons would be moving in orbits
about the nucleus just as the planets
do around the sun. Rutherford’s experiments suggested the size of the nucleus to be about 10–15 m to 10–14 m. From kinetic theory, the size of an atom was known to be 10–10 m, about 10,000 to 100,000 times larger than the size of the nucleus. Thus, the electrons would seem to be at a distance
from the nucleus of about 10,000 to 100,000 times the size of the nucleus
itself. Thus, most of an atom is empty space. With the atom being largely empty space, it is easy to see why most α particle go right through a thin metal foil. However, when α-particle happens to come near a nucleus, the intense electric field there scatters it through a large angle. The atomic electrons, being so light, do not appreciably affect the α-particles.
The scattering data can be analysed by employing
Rutherford’s nuclear model of the atom. As the gold foil is very thin, it can be assumed that α-particles will suffer not more than one scattering during their passage through it. Therefore, computation of the trajectory
of an alpha-particle scattered by a single nucleus is enough. Alpha-
particles are nuclei of helium atoms and, therefore, carry two units, 2e,
of positive charge and have the mass of the helium atom. The charge of
the gold nucleus is Z e, where Z is the atomic number of the atom; for
gold Z = 79. Since the nucleus of gold is about 50 times heavier than an
α-particle, it is reasonable to assume that it remains stationary throughout the scattering process. Under these assumptions, the trajectory of an alpha-particle can be computed employing Newton’s second law of motion and the Coulomb’s law for electrostatic
force of repulsion between the alpha-particle and the positively
charged nucleus.
3. ATOMIC SPECTRA
Each element has a characteristic spectrum
of radiation, which it emits. When an atomic gas or vapour is excited at
low pressure, usually by passing an electric current through it, the emitted radiation has a spectrum which contains certain specific wavelengths only. A spectrum of this kind is termed as emission line spectrum and it consists of bright lines on a
dark background. The spectrum emitted by atomic
hydrogen. Study of emission line spectra of a material can therefore serve as a type of “fingerprint” for identification of the gas. When white light
passes through a gas and we analyse the transmitted light
using a spectrometer we find some dark lines in the spectrum. These dark lines correspond precisely to those wavelengths which were found in the emission line spectrum of the gas. This is called the absorption spectrum
of the material of the gas.
4. B O H R MODEL OF THE HYDROGEN ATOM
The model of the atom proposed by Rutherford assumes that the atom, consisting of a central nucleus and revolving electron is stable much like sun-planet system which the model imitates. However, there are some fundamental differences between the two situations. While the planetary system is held by gravitational force, the nucleus-electron system being
charged objects, interact by Coulomb’s Law of force. We know that an object which moves in a circle is being constantly
accelerated – the acceleration being centripetal in nature.
According to classical electromagnetic theory, an
accelerating charged particle emits radiation in the form
of electromagnetic waves. The energy of an accelerating electron should therefore, continuously decrease. The electron would spiral inward and eventually fall into the nucleus. Thus, such an atom can not be stable.
Further, according to the classical electromagnetic theory, the frequency of the electromagnetic waves emitted by the revolving electrons is equal to the frequency of revolution. As the electrons spiral inwards, their angular velocities and hence their frequencies would change continuously, and so will the frequency of the light emitted. Thus, they would emit a continuous spectrum, in contradiction to the line spectrum actually observed. Clearly Rutherford model tells only a part of the story implying that the classical ideas are not sufficient to explain the atomic structure.
Comments
Post a Comment