NCERT Chapter 2 Science Class 10 Addition question solution || Acid, Base and Salt solution class 10 || Chemistry || Science ||
Page 18:
Ques 1: You have been provided with 3 test tubes. One of them contains distilled water and the other 2 contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Ans 1: If the colour of red litmus paper gets changed to blue, then it is a base and if there is no colour change, then it is either acidic or neutral. Thus, basic solution can be easily identified. Let us mark the three test tubes as A, B, and C. A drop of the solution in A is put on the red litmus paper. Same is repeated with solution B and C. If either of them changes colour to blue, then it is basic. Therefore, out of three, one is eliminated. Out of the remaining two, any one can be acidic or neutral. Now a drop of basic solution is mixed with a drop of each of the remaining two solutions separately and then the nature of the drops of the mixtures is checked. If the colour of red litmus turns blue, then the second solution is neutral and if there is no change in colour, then the second solution is acidic. This is because acidic and basic solutions neutralise each other. Hence, we can distinguish between the three types of solutions.
Page 22:
Ques 1: Why should curd and sour substances not be kept in brass and copper vessels?
Ans 1: Curd and other sour substances contain acids. Therefore, when they are kept in brass and copper vessels, the metal reacts with the acid to liberate hydrogen gas and harmful products, thereby spoiling the food.
Metal + Acid → Salt + Hydrogen gas
Cu + H Cl → Cu Cl 2 + H 2 ↑
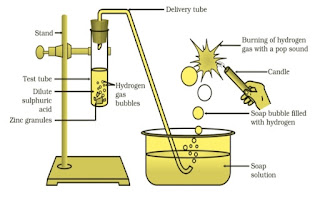
Ques 2: Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Ans 2: Hydrogen gas is usually liberated when an acid reacts with a metal.
Take few pieces of zinc granules and add 5 ml of dilute H 2 SO 4. Shake it and pass the gas produced into a soap solution. The bubbles of the soap solution are formed. These soap bubbles contain hydrogen gas.Ques 3: Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Ans 3: C a CO 3 + 2 H Cl → C a Cl 2 + CO 2 + H 2 O
Page 25:
Ques 1: Why do HCl, H NO 3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Ans 1: The dissociation of HCl or H NO 3 to form hydrogen ions always occurs in the presence of water. Hydrogen ions (H+) combine with H2O to form hydronium ions (H 3 O+) .
Ques 2: Why does an aqueous solution of an acid conduct electricity?
Ans 2: Acids dissociate in aqueous solutions to form ions. These ions are responsible for conduction of electricity.
Ques 3: Why does dry HCl gas not change the colour of the dry litmus paper?
Ans 3: Colour of the litmus paper is changed by the hydrogen ions. Dry HCl gas does not contain H+ ions. It is only in the aqueous solution that an acid dissociates to give ions. Since in this case, neither HCl is in the aqueous form nor the litmus paper is wet, therefore, the colour of the litmus paper does not change.
Ques 4: While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Ans 4: The process of dissolving an acid or a base in water is a highly exothermic one. Care must be taken while mixing concentrated nitric acid or sulphuric acid with water. The acid must always be added slowly to water with constant stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. The glass container may also break due to excessive local heating
Ques 5: How is the concentration of hydronium ions (H 3 O+) affected when a solution of an acid is diluted?
Ans 5: When an acid is diluted, the concentration of hydronium ions (H 3 O+ ) per unit volume decreases. This means that the strength of the acid decreases.
Ques 6: How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroxide?
Ans 6: The concentration of hydroxide ions (OH−) would increase when excess base is dissolved in a solution of sodium hydroxide.
Page 28:
Ques 1: You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic?
Ans 1: A pH value of less than 7 indicates an acidic solution, while greater than 7 indicates a basic solution. Therefore, the solution with pH = 6 is acidic and has more hydrogen ion concentration than the solution of pH = 8 which is basic.
Ques 2: What effect does the concentration of H+ ions have on the nature of the solution?
Ans 2: Concentration of H+ can have a varied effect on the nature of the solution. With an increase in H+ ion concentration, the solution becomes more acidic, while a decrease of H+ ion causes an increase in the basic city of the solution.
Ques 3: Do basic solutions also have H+ ions? If yes, then why are these basic?
Ans 3: Yes, basic solution also has H+ ions. However, their concentration is less as compared to the concentration of OH− ions that makes the solution basic.
Ques 4: Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Ans 4: If the soil is acidic and improper for cultivation, then to increase the basic city of soil, the farmer would treat the soil with quick lime or slaked lime or chalk.
Page 33:
Ques 1: What is the common name of the compound (calcium oxychloride) C a O Cl 2?
Ans 1: The common name of the compound C a O Cl 2 is bleaching powder.
Ques 2: Name the substance which on treatment with chlorine yields bleaching powder.
Ans 2: Calcium hydroxide [C a(OH)2], on treatment with chlorine Cl 2, yields bleaching powder.
Ques 3: Name the sodium compound which is used for softening hard water.
Ans 3: Washing soda (Na2CO3.10H2O) is used for softening hard water.
Ques 4: What will happen if a solution of sodium hydro carbonate is heated?
Ans 4: When a solution of sodium hydro carbonate is heated, sodium carbonate and water are formed with the evolution of carbon dioxide gas.
Ques 5: Write an equation to show the reaction between Plaster of Paris and water.
Ans 5: Plaster of Paris is a white powder and on mixing with water, it changes to gypsum giving a hard solid mass.



Comments
Post a Comment