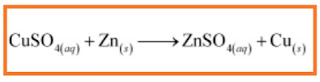
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.

Ques 14: In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved. Ans: «« Previous question Next question »»