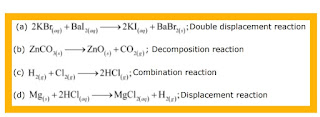
Write the balanced chemical equation for the following and identify the type of reaction in each case. (a)Potassium bromide + Barium iodide → Potassium iodide + Barium bromide(s)
Ques 8: Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a)Potassium bromide + Barium iodide → Potassium iodide + Barium bromide(s)
(b) Zinc carbonate→ Zinc oxide + Carbon dioxide
(c) Hydrogen + Chlorine→ Hydrogen chloride
(d) Magnesium + Hydrochloric acid → Magnesium chloride + Hydrogen
Ans 8:



Comments
Post a Comment