What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Ques 13: What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Ans 13: In a displacement reaction, a more reactive element replaces a less reactive element from a compound.
A + BX → AX + B, where A is more reactive than B
In a double displacement reaction, two atoms or a group of atoms switch places to form new compounds.
AB + CD → A D + BC
For example:
Displacement reaction:
Double displacement reaction:
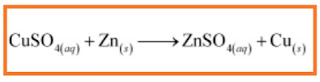



Comments
Post a Comment