NCERT Solutions class 10 science chapter 4 || Carbon and its compound solution|| Class 10 Chapter 4 Additional questions || Chemistry || Science ||
Page: 61
Ques 1: What would be the electron dot structure of carbon dioxide which has the formula CO2?
Ans 1: Electron dot structure of CO2 is
Ques 2: What would be the electron dot structure of a molecule of sulphur(S) which is made up of eight atoms of sulphur?
Ques 2: What are the two properties of carbon which leads to the huge number of carbon compounds we see around us?
Ans 2: The two most important features of carbon that give rise to the large number of compounds are as follows:
(i) Catenation: It is the ability to form bonds with other carbon atom. It is also known as self-linking property.
(ii) Tetravalency: Since, Carbon is having the valency of four.Hence, carbon is capable of bonding with four other atoms.
Ques 3: What will be the formula and electron dot structure of cyclopentane?
Ans 3: The formula for cyclopentane is C5H10. Its electron dot structure is:
Ques 4: Draw the structures for the following compounds.
Ans 4: (i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) HexanalQues 5: How would you name the following compounds?
Page: 71
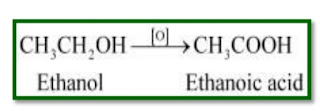
Ques 1: Why is the conversion of ethanol to ethanoic acid is an oxidation reaction?
Ans 1:
Since, the conversion of ethanol to ethanoic acid involves the addition of oxygen(O2) to ethanol. So, it is an oxidation reaction.
Ques 2: A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Ans 2:
When ethyne is burnt in air, it gives a sooty flame. This is due to the incomplete combustion caused by limited supply of air and produces yellow falme. However, if ethyne is burnt with oxygen, it gives a clean flame with temperature 3000°C because of complete combustion of oxygen. This oxy-acetylene flame is used for welding. It is not possible to attain such high temperature without mixing oxygen. This is the reason why a mixture of ethyne and air is not used.
Page: 74
Ques 1: How would you distinguish experimentally between an alcohol and a carboxylic acid?
Ans 1: We can differentiate between an alcohol and a carboxylic acid on the basis of their reaction with carbonates and hydrogen carbonates. Acid reacts with carbonate and hydrogen carbonate() to evolve CO2 gas that turns lime water milky.
Alcohols, on the other hand, does not react with carbonates(CO 3) and hydrogen carbonates.
Ques 2: What are oxidising agents?
Ans 2: Some substances such as alkaline potassium permanganate(KMnO4) and acidified potassium dichromate are capable of adding oxygen to the others. These are known as oxidising agents.
Page: 76
Ques 1: Would you be able to check if water is hard by using a detergent?
Ans 1: Detergents are ammonium(NH3) or sulphonate salts of long chain carboxylic acids. Unlike soap, they do not react with calcium(Ca) and magnesium(Mg) ions present in hard water to form scum. They give a good amount of lather irrespective of whether the water is hard or not. This means that detergents can be used in both soft water as well as hard water. Therefore, it not possible to check whether the water is hard or not with the help of detergent.
Ques 2: People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Ans 2: A soap molecule has two parts namely hydrophobic(chain part) and hydrophilic(carbon part). With the help of these, it attaches to the grease or dirt particle and forms a cluster called micelle. These micelles remain suspended as a colloid. To remove these micelles (entrapping the dirt), it is necessary to agitate the clothes.














Comments
Post a Comment