Nature of Cathode Rays
Nature of Cathode Rays
The following experiments help in understanding the nature of cathode rays:
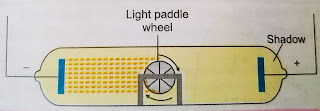
1. The cathode rays start from cathode and move towards anode. Whenever an object is placed inside the tube, it casts a shadow on the wall opposite to the cathode (in the figure below).During this experiment, the gas fluoresces only in regions outside the shadow. This experiment showed that the cathode rays travel in straight lines. Further, since the shadow falls on the wall opposite to the cathode, it shows that the ray travel from cathode towards the anode.(i) Effect of electric field. The effect of electric field on the cathode rays was studied by J. J. Thomson in 1897. The discharge tube was modified by adding a pair of metal plates as shown in figure below. When these plates are given opposite electric charges, the beams of cathode rays are deflected towards the positively charged plate. This shows that the particles in the cathode rays carry negative charge.
(ii) Effect of magnetic field. When the cathode rays are made to pass through a magnetic field, these are deflected in the direction corresponding to the presence of the negative charge on the particles. This is shown in figure below.
5.When the cathode rays are allowed to strike a thin metal foil, it gets heated op Thus, the cathode rays possess heating effect.
6. The cathode rays produce X rays when they strike against hard metals like tungsten, copper, etc.
7. The cathode rays upon striking glass or certain other materials cause them to glow produce fluorescence).
8. The cathode rays penetrate through thin sheets of aluminium and other metals.
9. The cathode rays affect the photographic plates.
10. The characteristics of cathode rays do not depend upon the nature of electrodes and the nature of gas present in the cathode rays, The ratio of charge to mass i.e., charge Mass is same for all the cathode rays irrespective of the gas used in the tube.
11. Cathode rays are negative in nature. So, it is deflected towards the positively charge electrode.






Comments
Post a Comment